No laughing matter – Nitrous oxide helps to unravel rapid antidepressant mechanisms
Ketamine has been intensively studied in psychiatry in recent years. Unlike traditional antidepressants, ketamine brings about its antidepressant effects very rapidly. A recent study conducted at the University of Helsinki, Finland, provides insights into the putative neurobiological mechanisms underlying rapid improvement of depression.
Since the discovery of the rapid-acting antidepressant effects of ketamine, a dissociative anesthetic drug used commonly in emergency medicine, researchers around the world have been trying to understand mechanisms underlying ketamine’s therapeutic effects. While several key components have been identified, the precise neurobiological basis remain unclear.
A small clinical trial published in 2015 suggest that nitrous oxide, another dissociative anesthetic, also possesses rapid antidepressant effects. The neurobiological mechanisms underlying the antidepressant effects of nitrous oxide were investigated in a preclinical study lead by Associate Professor Tomi Rantamäki, Ph.D. at the University of Helsinki. They show that nitrous oxide facilitates activation of the cortex of the brain during gas administration. A similar phenomenon has been previously associated with the mechanism of action of ketamine and electroconvulsive therapy (ECT). However, acute exposure of rodents to nitrous oxide produced negligible effects on several molecular changes intimately implicated in ketamine’s antidepressant effects.
“Activation of neurotrophic signaling has been causally connected to ketamine’s actions. The inability of laughing gas to trigger such changes was unexpected and puzzling,” says Rantamäki.
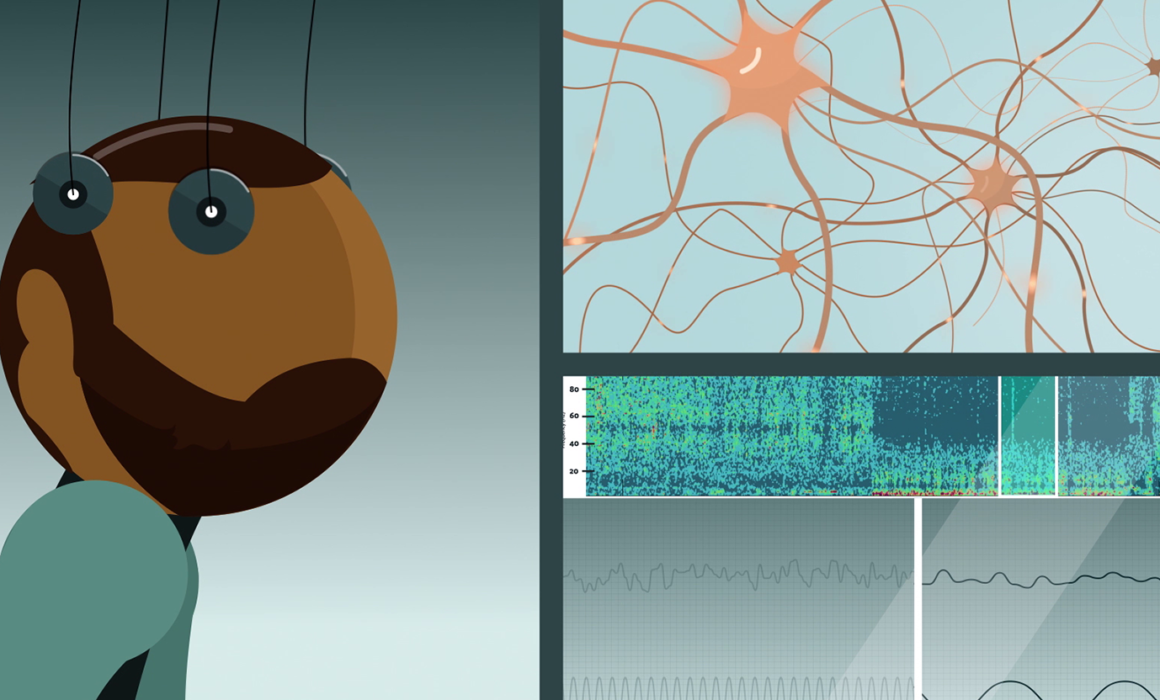
To understand this discrepancy, Rantamäki and his group studied the effects of the treatments on general brain activity using EEG. The immediate effects of nitrous oxide and ketamine were found to be quite different. A common factor for both treatments was, however, the gradual emergence of slow oscillations, characteristic of states of deep sleep. Intriguingly, these oscillations increased only after the peak of the drugs’ pharmacological effects had subsided.
“Once the drugs were eliminated from the brain and the body, a new wave of actions began. The initial excitatory effects were followed by a homeostatic increase in slow waves. A very similar phenomenon has been proposed to predict the onset of action of ECT,” explains Rantamäki.
Most importantly, the researchers were able to demonstrate for the first time that the activation of neurotrophic signaling pathways coincided with this period of increased slow EEG activity. While previous research has implicated neurotrophins and neuronal plasticity in the mechanisms of both traditional and rapid-acting antidepressant treatments, the link between slow EEG activity and neurotrophic signaling is new.
The mechanism of action of the prototypical rapid-acting treatment ketamine is commonly explained through specific alterations ingeniously controlled by the drug. Pharmacological effects are essential for any drug to bring its actions. However, these results propose that the core in rapid amelioration of depression may rely on intrinsic homeostatic changes triggered within the brain in response to pharmacological challenge and its consecutive release.
“Like throwing a rock into a still pond, the impact of the stone on the surface of the water sets the events in motion, but the subsequent waves linger on for a long time. We think that these waves tell us more than the initial impact,” says Samuel Kohtala, who is a Ph.D. student and the first author of the publication.
“Our observations provide evidence of a particular physiological brain state, which occurs similarly after these different treatments, and which we have found to coincide with specific molecular and electrophysiological signatures.” Rantamäki says.
The shared features of these treatments may provide scientists opportunities to develop measures to monitor and control the effectiveness of rapid-acting antidepressants. “Our discovery sheds light on the complex molecular mechanisms that are associated with rapid antidepressant effects of drugs such as laughing gas and ketamine. Furthermore, it supports the idea that some of the effects of rapid-acting treatments may be safely measured by using EEG or related methods, creating novel possibilities for therapy and drug development.”
While EEG has been used for decades in the study of neurophysiological mechanisms, no clear biomarkers have been found for CNS disorders. This time, the researchers think they have a strong biochemical correlate for an electrophysiological signal, but the work is still in its infancy.
“These findings are built upon decades of basic and clinical research related to the mechanisms of action of antidepressants,” says Kohtala. “The results beg the question of how these changes are ultimately linked to functional changes in the neuronal networks of the brain and whether the mechanisms involved have something to do with physiological slow oscillations, such as those occurring during sleep.”
Slow oscillations occur physiologically during the deepest stages of non-REM sleep, and have been suggested to be involved in important processes related to memory, repair and rejuvenation of the brain. Abnormalities in sleep and its quality are present in most cases of depression.
“To find out the right answers behind rapid antidepressant effects, we must tightly collaborate with sleep researchers and clinicians” concludes Rantamäki, whose team is now also part of the SleepWell multidisciplinary research program at the Faculty of Medicine, University of Helsinki.
Published first at Medical Xpress
See the full animation on YouTube